Kinetics of jet fuel (Ph. D. Project):
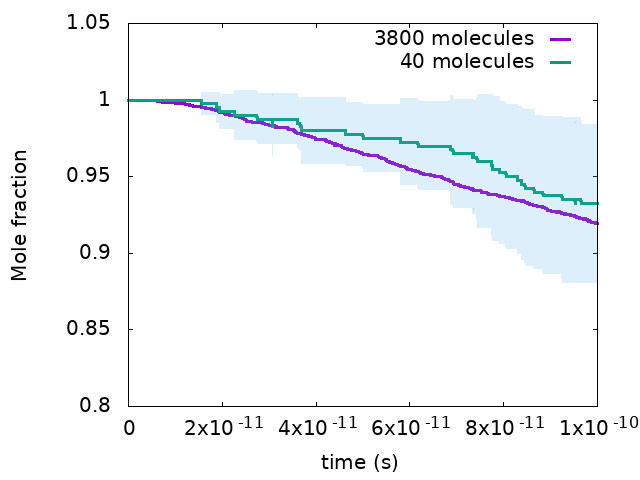 Fig: Comparison of JP10(Jet Fuel) concentration between a smaller and a large system simulation at T = 2000K, ρ = 0.20 kg/dm3. The shaded area indicates statistical deviations in ReaxFF simulations.
Fig: Comparison of JP10(Jet Fuel) concentration between a smaller and a large system simulation at T = 2000K, ρ = 0.20 kg/dm3. The shaded area indicates statistical deviations in ReaxFF simulations.
With the current advancement of materials research, new materials are being developed which can withstand extremely high pressures and temperatures. This will lead to the development of next generation high-pressure jet engines, the primary goal of which will be to increase both fuel efficiency and flexibility and decrease the carbon footprint. The existing chemical kinetic models of these fuels used to represent their combustion chemistry have not been developed considering these extreme operating conditions. In this work we are exploring the use of ReaxFF to investigate reaction dynamics of Jet Fuel as well as some of the renewable fuel candidates for Jet Engines at these extremely high pressures and temperatures.
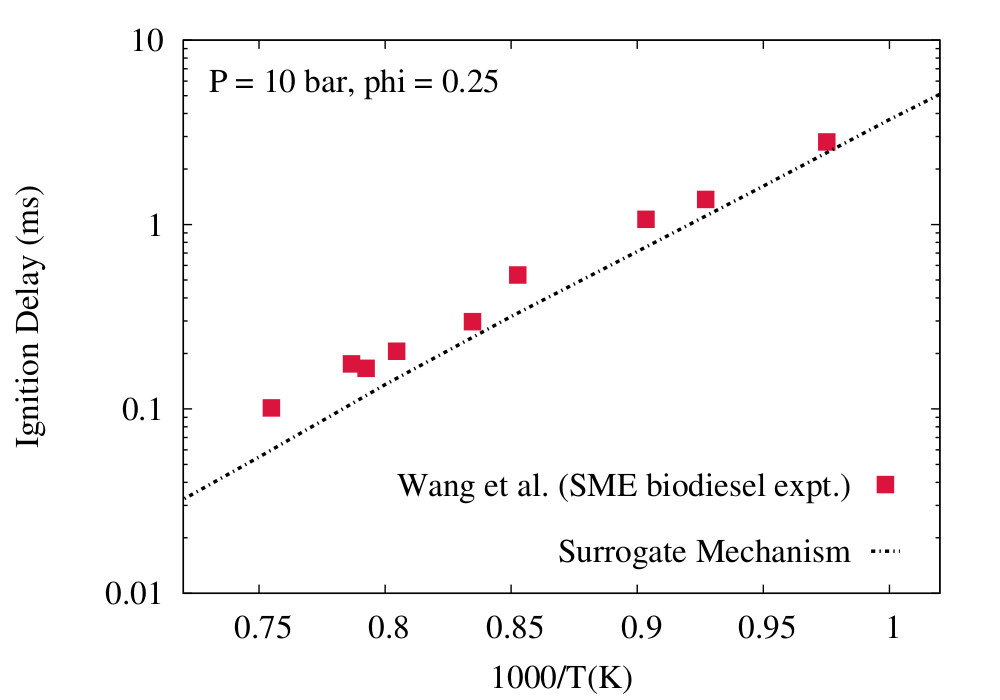 Fig: Ignition delays in a shock tube at P = 10 bar, phi = 0.25; symbols: experiments for biodiesel, solid lines: Surrogate
(presented at the 10th U.S. National Combustion Meeting, 2017)
Fig: Ignition delays in a shock tube at P = 10 bar, phi = 0.25; symbols: experiments for biodiesel, solid lines: Surrogate
(presented at the 10th U.S. National Combustion Meeting, 2017)